About QMR Blasting Analysis
Thank you for choosing QMR Blasting Analysis for your reactive ground testing. QMR Blasting Analysis is an Australian owned business headquartered in Brisbane, Australia. QMR Blasting Analysis is proudly independent and is not affiliated with any explosives manufacturers. We specialise in reactive ground and explosive testing and aim to provide you with accurate, trusted data so that you can blast safely and confidently. If you have any queries or would like to better understand the results in this report, please don’t hesitate to contact us.
AEISG Code Test Compliance
Date of this test: April 2025
dISCLAIMER
The contents of QMR Blasting Analysis Pty Limited documents are for general information only. The information upon which the analyses in Queensland Magnetic Research Pty Ltd documents are based has been either partly or entirely sourced from other parties. The reliability of these sources cannot be absolutely proven and Queensland Magnetic Research Pty Ltd does not represent or warrant that the information is correct.
The contents of Queensland Magnetic Research Pty Ltd documents may be inter-related and consequently invalid if considered individually or out-side of context of the over-all situation. The principals and employees of Queensland Magnetic Research Pty Ltd do does not accept any liability for any claim arising out of or in connection with any reliance on the information or the derived analyses, conclusions or recommendations contained in Queensland Magnetic Research Pty Ltd.
Before using the information or recommendations contained in Queensland Magnetic Research Pty Ltd documents in a particular situation it is essential that, amongst other things, the following criteria be taken into account:
• Whether the particular technique proposed to be used is appropriate for the circumstances;
• Whether the persons using it have the necessary competency and experience;
• The environmental conditions in which it is to be used;
• The specific aims intended to be achieved and whether those aims are achievable in the particular circumstances; and
• The sequence of steps which need to be followed in the particular circumstances.
Reactivity and Risk Management
Explosive reactivity testing evaluates the reaction, if any, between your rock samples and ammonium nitrate. Reactive ground is caused by sulphides and products of sulphides react with the nitrates in ammonium nitrate. This can result in a runaway reaction resulting in unplanned detonation. Reactive ground is a principal hazard that must be controlled appropriately. Comprehensive and regular testing of the rock mass is a critical step in understanding your rock mass and explosive compatibility.
To manage this risk, the testing method and frequency is described in The Australian Explosives Industry Safety Group Inc (AEISG) Code of Practice – Elevated Temperature and Reactive Ground. The provided samples for testing should have been collected in accordance with this code, including (code section):
• The minimum recommended number of samples selected and collected for testing is 12 (7.3)
• Each sample is at least 0.5 kg in size and all rocks must be smaller than 50mm in diameter (7.2.1)
• The samples are selected with positive bias for reactivity, from strata that are known to contain sulphides (7.3)
• The samples are fresh, and their location is known and surveyed (7.1)
• Testing is performed annually (7.4.2)
• If a change has occurred, additional testing is conducted (8.3.2)
More information about reactive ground is listed in Appendix B – Reactive Ground Processes.
Reactivity Testing
Ammonium nitrate-based explosives can react with sulphides, resulting in an auto catalysing reaction that can result in an unplanned and uncontrolled detonation. Reactivity testing is an important step in understanding the mineral composition of your ground and the propensity that ground to react with ammonium nitrate. Knowing the reactivity of your ground is an essential step in evaluating and mitigating the risks of blasting.
There are multiple tests that can be undertaken to evaluated reactivity. The AEISG is an industry association that represents the interest of suppliers and manufacturers of explosives in Australasia. Members of the AEISG must comply with all AEISG codes of practice and policies. To ensure that your explosive provider remains compliant with their obligations, the tests prescribed in the AEISG Code of Practice – Elevated Temperature and Reactive Ground, are recommended as a baseline. There are two types of tests listed in the Code:
• Reactivity Screening; and
• Product Selection Testing.
Reactivity Screening are tests that identify whether ground is reactive towards ammonium nitrate. The most common test is the Isothermal Reactive Ground Test (IRGT) which was created for screening reactive samples and for carrying out sleep time analysis. If the ground is found to be reactive in the screening test, product selection testing is performed to evaluate the performance of an inhibited product when exposed to the reactive ground.
Reactive ground testing is not limited to and should not be limited to the tests listed in the Code. The QMR Test provides valuable insight into why a test may pass or fail the IRGT. The QMR test evaluates environmental attributes that may inhibit or accelerate reactivity. If the screening test result is not reactive, it is good to know if that outcome was influenced by the presence of a natural inhibitor that may only be present in local lithologies. A different part of your mine with different lithology may have a different reactivity result. The AEISG Test methodology is listed in the AEISG Code of Practice - Elevated Temperature and Reactive Ground.
Isothermal Reactive Ground Test Results
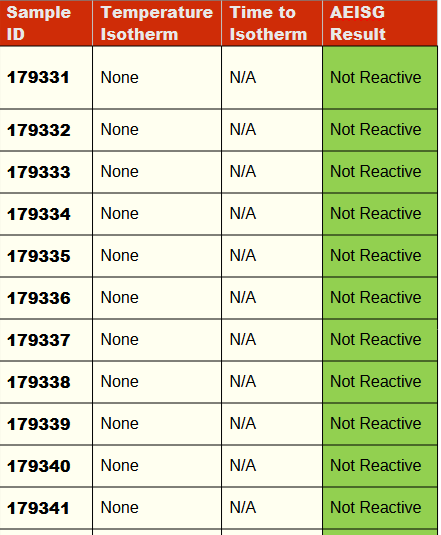
The results for the screening test are shown in Table 1. The AEISG classifies a sample as reactive if it exceeds the background temperature of the sample by 2 degrees centigrade or more throughout the entire test duration. It is difficult to determine if a difference of 2 degrees has occurred during the heating phase of the test. The temperature trace for each sample is studied independently to observe for any evidence of an exothermic reaction. Charts of the sample temperature traces are shown in Appendix A.
Table 1: Screening Test Results
All 13 tests were not identified to be reactive in accordance with the AEISG Code.
QMR Reactive Ground Testing
The QMR Test incorporates the role of environmental factors that could impact the reactivity result. Coal and carbonates can act to inhibit the reaction. The pH is measured, as acidity is strongly correlated with reactivity. As the reaction between ammonium nitrate and sulphides is caused primarily reactive sulphides, the presence of reactive sulphides is tested. The results from the QMR Test are listed in with only sample 179340 containing more than trace reactive sulphide with coal inhibiting a reaction. This sample is a risk for a hot ground event (refer to risk Matrices).
Table 2: QMR Test results
Conclusion
The samples provided for testing were not reactive in accordance with the requirements of the AEISG Isotherm Test and QMR’s Reactive Ground Test.
Analysis by: GC
Report approved by: Dr Gary Cavanough
Appendix A – Isotherms
Isothermal Reactive Ground Tests
Appendix B – Reactive Ground Processes
Sulphides are typically present at pyrite, however, can be found in other minerals. In the presence of moisture, pyrite will weather, forming reagent products. Water, as humidity or as a solution, is necessary for the reaction to occur. In a cool dry environment, a reaction between ammonium nitrate prill and pyrite may not occur at all.
In a mining environment there will be multiple minerals and chemicals that act to inhibit or catalyse the reactivity of ammonium nitrate and sulphides. While water is necessary for the reaction to occur, too much water can dissolve the reactant products and dissipate heat, slowing or preventing the reaction from occurring. It is important to understand the reactions that are taking place to best apply your test results to your site, however it is prudent from a risk management perspective to assume the worst-case environment and mitigate the risk of a reactive ground event via comprehensive risk management.
Weathering
Reactive ground isn’t as simple as identifying specific minerals. Weathering of minerals, including via bacterial oxidation can result in unexpected chemical products. This is explained concisely explained by the USBM (1979). Pyrite (FeS2), oxygen and water react to form a positively charged ferrous ion, a negatively charged sulphate ion and a positively charged hydrogen ion.
Where pyrite exists, it is therefore possible that the following reagents will have accumulated: pyrite, sulfuric acid, ferric and ferrous sulphates. This is the same process that causes acid my drainage.
Induction
When ammonium nitrate is introduced into a reactive environment, it will react with iron sulphides, ferrous ions and sulphuric acid to form nitric oxide and ferric ions. The reaction zone will become more acidic and as the redox reaction is exothermic, the temperature will increase.
Intermediate
In this stage the primary source of the reagents supporting the reaction are no longer environmental. They are provided by the products of the induction stage reaction. Nitric acid and ferric ions, react with pyrite to create the reagents to catalyse the reaction with the ammonium nitrate. Temperatures may exceed the initiation temperature of detonators.
Ignition
In the case of hot and reactive ground, or reactive ground with low thermal loss, the temperature of the bulk explosive may exceed 500°C and detonate via the following path:
Decomposition of Ammonium Nitrate to ammonia gas and nitric acid:
NH4NO3 ↔ HNO3 + NH3 Equation 1
Ammonia has much higher vapour pressure than nitric acid and the melt self-acidifies increasing the heat of reaction with any sulphide at temperature > 150°C.
Cracking off hydrogen from the ammonia gas due to temperature:
2NH3↔N2 + 3H2 Equation 2
The ammonia gas cracks off hydrogen at approximately 500°C and ignition and/or detonation of hydrogen causes shock initiation of ammonium nitrate that has been sensitized by hydrogen gas and heat induced violent mixing or ignition and detonation of hydrogen causes shock.
Factors that affect reactivity are listed below (Kennedy and Tyson, 2001).
Reactive Ground on a hotplate at 160°C showing hydrogen flame
Rumball (1991) also notes that host minerals may interact with the reagents and products or affect the heat flow during self-heating reactions. Examples include the buffering of pH by carbonates, clays and feldspars, and the adsorption of heat by the dehydration of hydrated minerals. Other important properties of the reactive environment include the ability of minerals to transfer heat, and their rate of temperature increase as heat is applied. The QMR test was developed to investigate the common environmental factors that may impact a screening test outcome.
The QMR test identifies the presence of reagents that drives the reactivity, reactive sulphides, and the acidity of the reaction. The presence of common minerals that may react with the reagents or reaction products are also identified, including coal and carbonates. Coal and carbonates can increase the pH resulting in a reduction in the reaction rate. This reduction can range from mild to stopping the reaction entirely. It is important to understand why screening test may return a positive or negative result, as a change in lithologies in different areas of your mine can result in an adverse reactive environment if the presence of minerals that inhibit the reaction are lost.
Risk Matrices
References
Anon. (2020). Code of Practice Elevated Temperature and Reactive Ground. Australia Explosives Industry and Safety Group Inc. AEISG.
Anon (2022). Weekly Incident Summary. NSW Resources Regulator. Week ending Friday 28 January 2022
Cavanough G. (2023) “A New Method of Reactive Ground Testing” Cleveland: ISEE Conference Details
Chief Dangerous Goods Officer (2022),” Ammonium nitrate emulsion tanker trailer explosion”, Incident Alert. Government of Western Australia Department of Mines, Industry Regulation and Safety.
Babrauskas V. & Legger D. (2019), “Thermal decomposition of ammonium nitrate” Fire and Materials Wiley pp250-268
Bauer, A. (2015) SAFES Newsletter NO53 June 2015 citing:
Bauer A, A King. 1979. Critical initiation parameters for molten Ammonium Nitrate. Kingston: Canadian Fertilizer Institute.
Shah, Kish. 2009. "Summary of the work at Queens University by Bauer King & Heater." ANNA (AMMONIUM NITRATE AND NITRIC ACID). Little Rock AR: ANNA.
Briggs T., Kelso I. (2001) “Ammonium Nitrate-Sulfide Reactivity at the Century Zn-Pb-Ag Mine, Northwest Queensland, Australia” Explor. Mining Geol., Vol. 10, No. 3, 2001
Brill T. B., Brush P.J.& Patil D. G. (1993). “Thermal decomposition of energetic materials 58. Chemistry of ammonium nitrate and ammonium dinitramide near the burning surface temperature.” Combust Flame.1993;92:178-186.
Downs G, (2014), “Ammonium nitrate explodes during transport incident” Explosives safety alert no. 86 | 09 October 2014 | Version 1, Queensland Department of Natural Resources and Mines
Han X., Freij S., Feng S., Gunwaan R., Zhang D. and Waters P. (2005),"A Chemical Characterisation of Pyritic Black Shales in an Iron Ore Mine" Fremantle, WA, 19 - 21 September 2005 Iron Ore Conference
Gunsinger, M.R., Ptacek, C.J., Blowes, D.W, and Jambor, J.L, (2006) "Evaluation of long-term sulfide oxidation processes within pyrrhotite-rich tailering, Lyn Lake, Manitoba", Contaminant Hydrology 83, 2 149-170
Gunawan, R. & Zhang D (2009). Thermal stability and kinetics of decomposition of ammonium nitrate in the presence of pyrite. Journal of Hazardous Material 165 (pp. 751-758). Elsevier
Harries, G., Bellairs, P., Scott Stewart, J., (1986), "The Reaction of Ammonium Nitrate with Black Pyritic Shale at Mt Whaleback", AusIMM/IE Large Open Pit Mining Conference 141-146.
Kiiski, H. (2009). Properties of Ammonium Nitrate based fertilisers, Dissertation for the Degree of Doctor Philosophiae. University of Helsinke, Faculty of Science, Department of Chemistry Helsinki Finland
Kennedy B., and Tyson N. (2001), “Blasting in Reactive Ground”, Proceedings EXPLO 2001, p55-61.
Littlefair M., Rounsley R., Beach F., Jassini D. and Pugh G (2003), "Reactive Ground and Ammoniun Nitrate Explosive Interactions", Fifth Large Open Pit Mining Conference. Kalgoorlie WA 3-5 November
Lukaszewski, G. (1968*“The Reaction of ANFO Explosives with Mineral Sulfides”, Proc.Aust.Inst. Min.Met 228, December
Miron et al, (1983) “Report of Investigations 8727 Reactivity of ANFO With Pyrite Containing Weathering Products Evaluation of Additional Inhibitors”.
Priyanandra, A., Djerdjev, A., Gore, G., Neto, C., Beattie, J. & Hawkett, B. (2015). Thermal stability and kinetics of decomposition of ammonium nitrate in the presence of pyrite. Journal of Hazardous Material 283 (pp. 314-320). Elsevier
Proulx, R. and Scott, N, (2000) "Drilling and Blasting in Hot and Reactive Ground Conditions at Barrick Goldstrike’s Meikle Mine” International Society of Explosives
Rumball J. A. (1991), “The Interaction of Partially Weathered Sulphides in the Mt McRae Shale Formation with Ammonium Nitrate”, PhD Thesis, Murdoch University Engineers 2000G Vol. 1 - Drilling & Blasting in Hot and Reactive Ground Conditions - P 319
Ramirsz, M., Reyes, A. and Alarcon A. (2018) “Improvement of Safety Practices for Loading of ANFO-based Explosives in Zones with Reactive Rocks in Chile” ISEE Engineers 2000G Vol. 1 - Drilling & Blasting in Hot and Reactive Ground Conditions - P 319
Ramirsz, M., Reyes, A. and Alarcon A. (2018) “Improvement of Safety Practices for Loading of ANFO-based Explosives in Zones with Reactive Rocks in Chile” ISEE
Wang G, Chen C, Beshiwork B.A & Lin B (2023) “Developing a low-carbon hybrid of ammonia fuel cell and internal combustion engine for carbon neutrality” Elsevier Applications in Energy and Combustion Science